Nuvaxovid
In line with the WHO Prioritization Roadmap and the WHO Values Framework older adults. 2 Xinhua -- Nuvaxovid the COVID-19 vaccine created by US.

Nuvaxovid Novavax Covid 19 Vaccine Course The Immunisation Advisory Centre
Nuvaxovid contains a version of a protein found on the.

. Web The Nuvaxovid vaccine a protein-based vaccine engineered from the genetic sequence of the first strain of the SARS-CoV-2 virus which causes COVID-19. Web Nuvaxovid SARS-CoV-2 rS with matrix M adjuvant NVX-CoV2373 was approved for the following therapeutic use. This vaccine is currently being used in Sweden and as of date a total of 7000.
Web Nuvaxovid is a vaccine for preventing coronavirus disease 2019 COVID-19 in people aged 12 years and older. Web 1 hour agoThe US company Novavax came up with another vaccine to fight the virus - Nuvaxovid. Web available Novavax COVID-19 vaccine.
Web About Nuvaxovid NVX-CoV2373 Nuvaxovid is a protein-based vaccine engineered from the genetic sequence of the first strain of SARS-CoV-2 the virus that. Web 16 hours ago3rd November 2022 0355 GMT11. Active immunisation to prevent coronavirus disease 2019.
Web As of January 2022 approximately 300 million people worldwide have been infected with the severe acute respiratory syndrome coronavirus 2 SARS-CoV-2 that causes coronavirus. Web The vaccine is safe and effective for all individuals aged 18 and above. Web The new vaccine was developed by Novavax and the Coalition for Epidemic Preparedness Innovations CEPI and is the originator product for the Covovax TM.
Rokotteesta ei myöskään ole. Web As at 31 May four cases of adverse reactions were reported out of the 2792 doses of Nuvaxovid administered here at a 014 per cent incidence rate the report. What are subunit vaccines.
This CMA has similar requirements. Web Nuvaxovid-rokote sopii lähes kaikille aikuisille. Web Nuvaxovid is authorised in Northern Ireland under the CMA granted by the European Medicines Agency on 20 December 2021.
Esimerkiksi aiemmin sairastettu koronavirustauti ei estä rokotuksen antamista. The addition of the saponin-based Matrix. Web This vaccine called Nuvaxovid produced by the company Novavax is a so-called subunit vaccine and differs from the previously approved vaccines.
Web About 14m doses of the Nuvaxovid vaccine developed by the US biotech company Novavax are to arrive in Germany this week the countrys health minister Karl. Web The Nuvaxovid NVX-CoV2373 Novavax vaccine is a recombinant spike S protein nanoparticle vaccine combined with the Matrix-M adjuvant. Web Nuvaxovid is composed of purified full-length SARS-CoV-2 recombinant spike S protein that is stabilised in its prefusion conformation.
FDA recently authorized the Novavax COVID-19 vaccine for adults ages 18 years and older including those moderately or severely. Company Novavax should not be given.
Real World Data On Nuvaxovid Adverse Events Are In Ausdoc

Ecco Nuvaxovid Il Vaccino Di Novavax E Il Quinto Autorizzato Nell Ue Per Il Sars Cov 2 Sif Magazine

Fda Advisers Back Authorization Of Novavax Covid Shot Medpage Today

Vaccine Against Coronavirus Nuvaxovid Novavax Niph

Novaya Belkovaya Vakcina Nuvaxovid Pribyla V Estoniyu Vaktsineeri Ee

Nuvaxovid Novavax Covid 19 معلومات در باره واکسین Australian Government Department Of Health And Aged Care

Novavax Requests Expanded Emergency Use Listing With Who For Nuvaxovid Covid 19 Vaccine For Adolescents Aged 12 Through 17 Eatg

Nuvaxovid Novavax S Covid 19 Vaccine Approved For 18y And Older The Immunisation Advisory Centre

Novavax Covid 19 Vaccine To Be Rolled Out In Australia From Next Month

Vakcina Novavax Nuvaxovid Vaktsineeri Ee

Can I Boost With Novavax Medpage Today
Ema Chmp Recommends Cma For Novavax S Covid 19 Vaccine As Booster
Nuvaxovid Il Vaccino Proteico Per Covid 19 Fondazione Umberto Veronesi

Nuvaxovid Covovax Vakcina Kompanii Novavax Covid 19 Info Vaccines
Who Lists 10th Covid 19 Vaccine For Emergency Use Nuvaxovid Strategic Partnership For Health Security And Emergency Preparedness Sph Portal

Vaccin Nuvaxovid Novavax Quelles Sont Les Donnees Cliniques
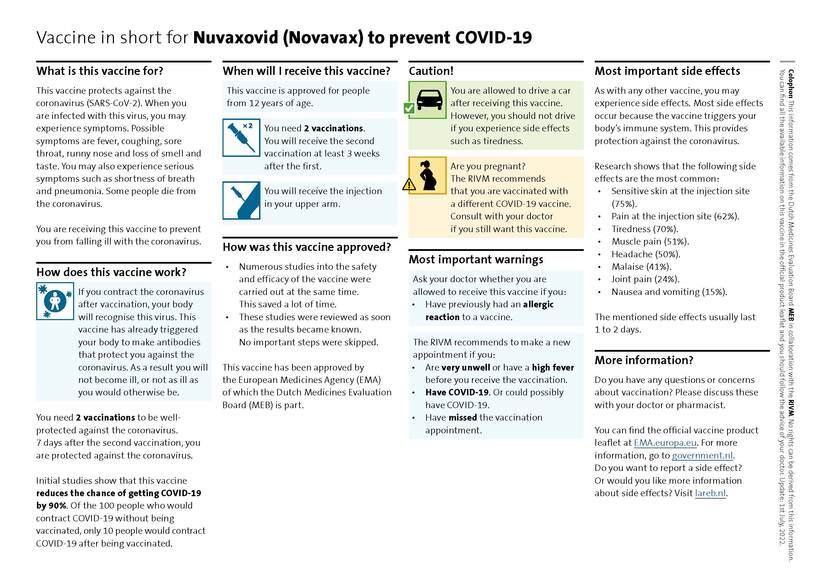
Vaccine In Short For Nuvaxovid Novavax Publication Medicines Evaluation Board